To reduce the number of incidents related to medicines -Toward that end
Takao Orii1,2*, Ryo Suzuki2,3, Tomofumi Yamaguchi3, Tadasato Sugita3
1Medical informatics, NTT Medical Center, Tokyo 141-8625, Japan
2Department of pharmacy, NTT Medical Center, Tokyo 141-8625, Japan
3Patient Safety, NTT Medical Center, Tokyo 141-8625, Japan
ABSTRACT
Objective: With the revision of the Pharmaceutical Affairs Law, it is required to ensure traceability by utilizing barcodes. Methods and Results: In this paper, we describe the usefulness of barcodes on dispensing and packaging units in preventing drug-related incidents, and the need to systemize the use of barcodes. Although it is necessary to establish and standardize a system to utilize barcodes on dispensing and packaging units of medicines, it is necessary to consider the establishment and development of a system that leads to the sharing of information based on the collaboration among departments, because the time and effort required to read barcodes increases and the introduction of equipment is costly. In this study, we will build a system and develop it. Conclusion: In this study, although the construction and deployment of the system has not been implemented, the current operations were analyzed quantitatively from the analysis of incident reports. Since many of the tasks that require priority consideration are bedside or standing tasks, it is necessary to select barcode readers that do not interfere with normal operations, such as those that are lightweight or easy to operate without wires, and to create operational procedures.
Keywords: Medicines; Incidents; Barcodes
Corresponding author:
Takao Orii, E-mail: orii-tky@umin.ac.jp
For reprints contact: reprints@sppub.org
Received 23 June 2022; Accepted 04 August 2022; Available online 28 September 2022
INTRODUCTION
With the recent progress and spread of medical information and communication technology, barcodes and electronic tags are being used in all aspects of daily life.[1,2,3] However, at present, compared to the use of barcodes and electronic tags in the settlement of accounts for food and general merchandise, in international distribution systems, and in public facilities, the use of barcodes and electronic tags for pharmaceuticals in the medical field has reached the stage of practical application, but is not yet sufficient.
OBJECTIVES
The purpose of this study is to clarify where barcodes should be applied in the life cycle of pharmaceutical products, from the pharmaceutical company to the pharmaceutical wholesaler, and then to the patient at the medical institution, especially in the areas of quality control and safety management of pharmaceutical products. In this study, we focused on the use of barcodes in the medical field, and on the use of barcodes in the results obtained from the analysis of incident reports. In addition, we discussed issues related to the use of barcodes that are necessary for the construction of systems for incident prevention.
METHODS
The study period was from October 2019 to March 2021. The subject of the survey was incident reports submitted to the Medical Safety Management Office of NTT Medical Center Tokyo (hereafter referred to as “the hospital”). Among them, reports related to medicines, such as barcodes, labels, and collation, were included in the study.
RESULTS
The total number of incident reports during the study period was 8,708. Of the total number of reports, 579 (about 7%) were related to medicines such as barcodes, labels, and matching, which were the subject of this study. Of these, 160 (about 28%) were reports of incidents that could have been prevented if barcodes had been used. Of these 160 reports, 80 were for injectable drugs and 80 were for oral and topical drugs (Figure 1).
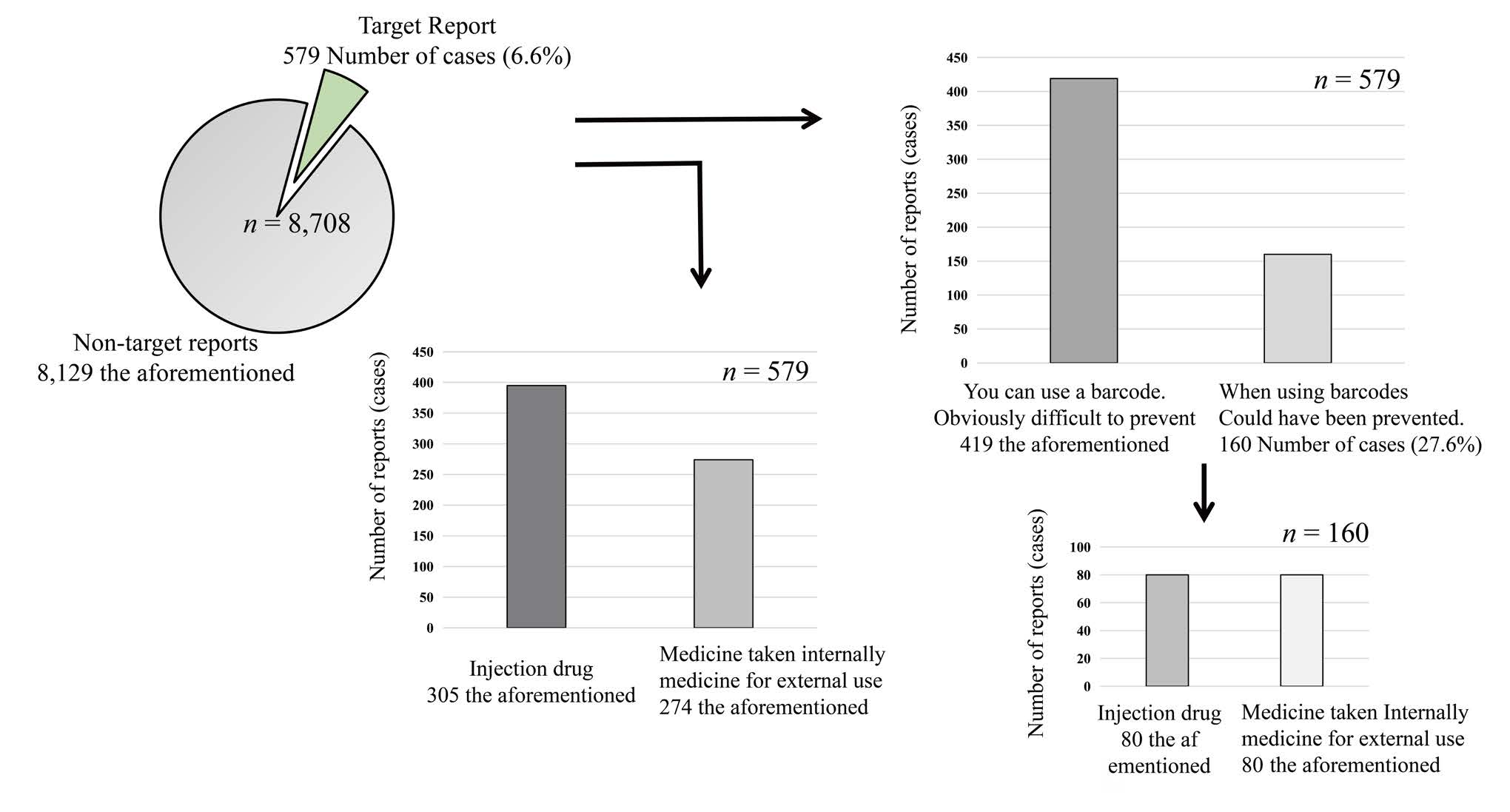
Figure 1: Simulated use of pharmaceutical barcodes
In the top 10 incident reports of all 8,708 reports, “contents related to drugs” was the most common item with 1,967 reports (about 23%), and the same was true for the three occupational categories of physicians, nurses, and pharmacists (Figure 2).
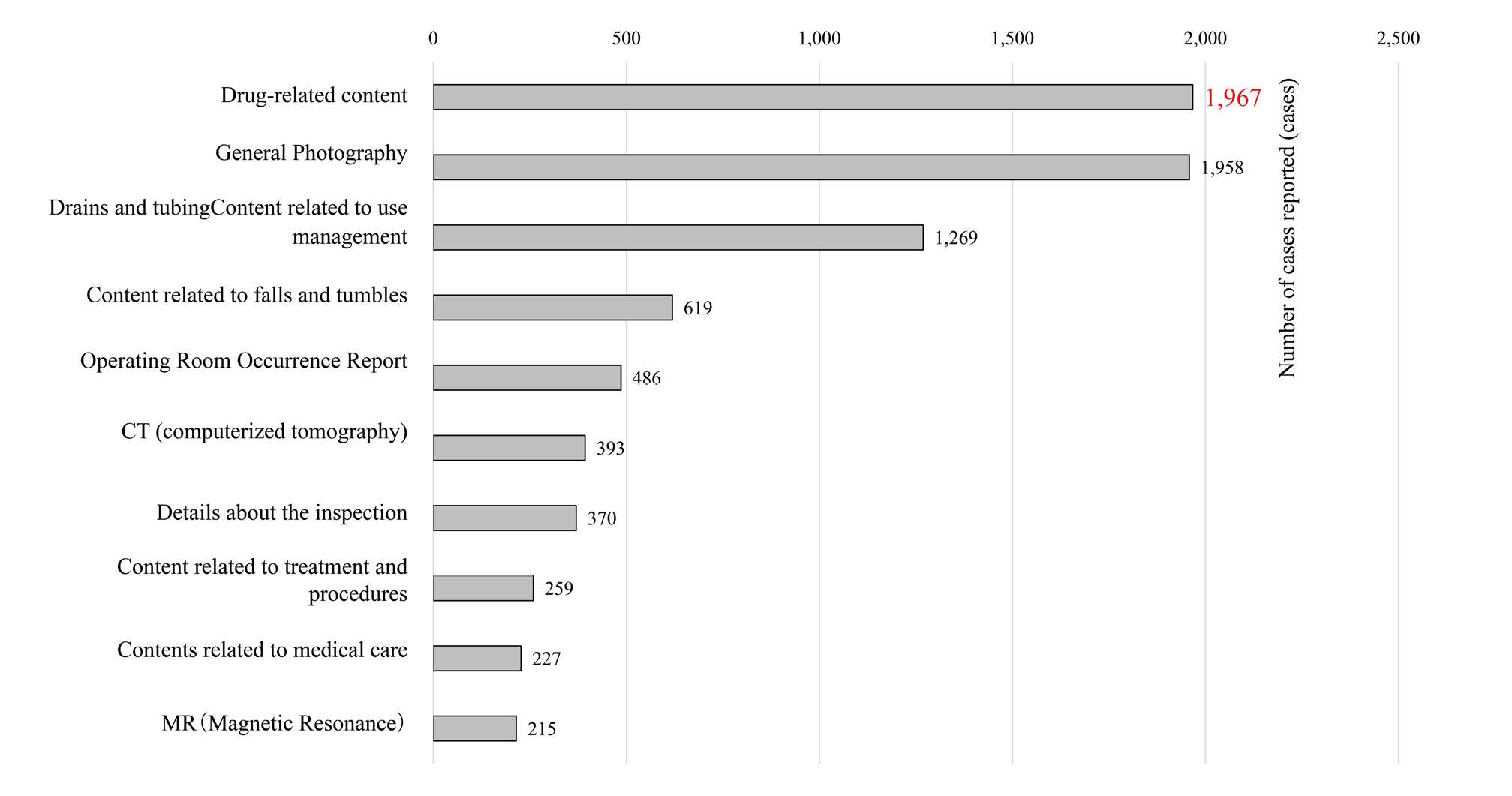
Figure 2: Total number of reports during the survey period (top10 items)
The 160 reports that could have been prevented if the barcodes of medicines had been used were analyzed by level (Figure 3). Of the 160 reports, 108 (about 68%) were reports of Level 1 or higher that were implemented on patients (Table 1).
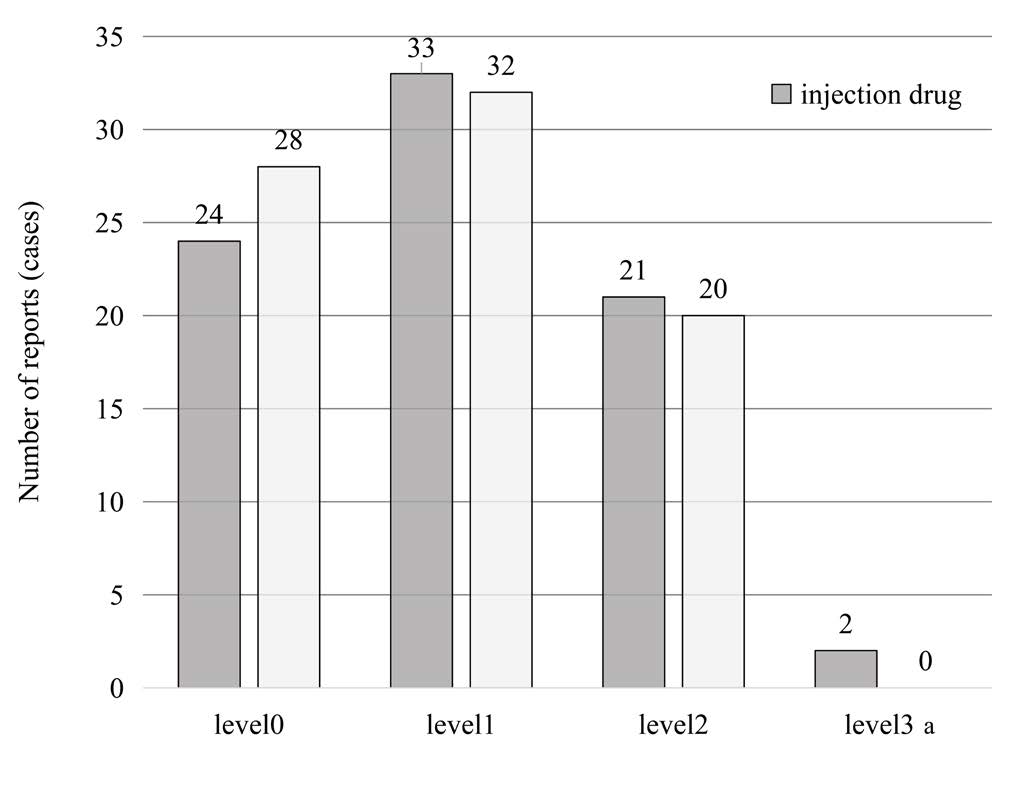
Figure 3: Number of incidents reported by incident level
| Level | Continuity of Injury | Degree of Injury | Contents |
| Level5 | Death | – | Death (excluding death due to the natural course of the original disease) |
| Level4b | Persistent | Moderate to severe | Permanent disability or residual disability, with significant functional impairment or cosmetic problems |
| Level4a | Persistent | Moderate to severe | Permanent disability or sequelae, but not accompanied by significant functional impairment or cosmetic problems |
| Level4a | Persistent | Moderate to severe | Permanent disability or sequelae, but not accompanied by significant functional impairment or cosmetic problems |
| Level3b | Transient (pain or fever) | Altitude | Required advanced procedures or treatment (e.g., severe changes in vital signs, placement on a ventilator, surgery, extended hospital stay, outpatient admission, bone fracture) |
| Level3a | Transient (pain or fever) | Moderate | Required simple procedures or treatments (disinfecting, compressing, suturing skin, administering painkillers, etc.) |
| Level2 | Transient (pain or fever) | Slight degree | No treatment or therapy was given (the need for increased patient observation, minor changes in vital signs, and tests to confirm safety arose) |
| Level1 | None | – | There was no actual harm to the patient (but we can’t deny that it may have had some effect) |
| Level0 | -Mr. | – | Errors and drug/medical device malfunctions were observed, but not implemented on the patient |
DISCUSSION
The analysis of drug-related reports is very important in considering medical safety. The Law for Partial Revision of the Law for Securing the Quality, Efficacy and Safety of Drugs and Medical Devices (the Revised Pharmaceutical Affairs Law) was promulgated in December 2019. This amended Pharmaceutical Machine and Device Law is related to barcode labeling for traceability in December 2022. Thus, the application of barcodes to the medical field is gradually progressing.
In hospitals, a variety of incidents can occur every day. Although each facility maintains manuals, compliance, and education in order to reduce the number of incidents, there is a limit to what can be done by itself.[4,5,6,7]
In this study, we targeted the incident contents related to barcodes of medicines, especially injectable drugs.
Of the 160 reports that could have been prevented using barcodes, 108 were conducted on patients, but fortunately no obvious health hazards to patients were identified.
As for the results of injectable drugs, there was a report of a drug being mistakenly mixed with a saline solution, which caused a change in formulation, instead of the dextrose solution (infusion solution) specified in the instructions. In addition, there were reports of contrast media that were prepared differently from the instructions and administered unnoticed during contrast media testing. In addition, there was a report that an IV label was mistakenly attached to an antibiotic that was to be administered to another patient, and the patient thought that the antibiotic had been prepared and administered differently from the instructions, resulting in a missed dose. It was suggested again that it is difficult to stop an error once it has been missed, as in the Swiss cheese model, with the intervention of another person.
For injectable drugs, the use of barcodes on the dispensing packaging unit will enable reliable verification and traceability from the time the drug enters the warehouse to the time it is used by the patient and even to the time it is disposed of.
The results of this survey show that there are some cases in which it is difficult to use barcodes on the dispensing packaging unit when reporting oral and topical medications. Examples of such cases are when the drug is removed from the press through pack (PTP) sheet for packaging or crushing, or when the drug is separated from the PTP sheet for dispensing or distributing and the barcode is not displayed.
This study suggests that the use of barcodes on the dispensing packaging unit, which enables reliable verification and recording of medications, is useful in preventing incidents. Although the importance of barcodes has been understood for a long time, few facilities have implemented them sufficiently. For such cases, the use of barcodes for each dispensing package will enable accurate matching and prevent manual errors, and if the information can be captured during audits, it will enable accurate recording and traceability. If the information can be captured during an audit, accurate records and traceability will be possible. Instead of having to examine business procedures, create business procedure manuals, and increase work and costs, reliable verification will be possible. In addition, it is necessary to educate the staff to understand that human intervention will help prevent errors.
The use of pharmaceutical barcodes can ensure patient safety by providing access to the latest digital information. The use of pharmaceutical barcodes by healthcare professionals and their supply chain partners should be standardized to prevent the risk of misidentification and to establish a system to ensure patient safety.
CONCLUSION
In the future, the use of barcodes on dispensing and packaging units, Global Trade Item Number (GTIN), and other barcodes in the medical field will be essential for preventing incidents. The establishment of a system in which the serial number and expiration date of a drug product are printed on the barcode of the dispensing package unit and can be utilized will enable traceability from upstream to downstream, from receiving to dispensing in the hospital, and from use on the patient to disposal. The establishment of such a system will not only establish safety management related to pharmaceuticals, but will also have a significant effect on operational efficiency.
*GTIN: GS1 barcodes always contain a code GTIN to identify the packaging of the pharmaceutical product. GTIN is an international standard product identification code and is designated so that it does not overlap internationally. The GTIN is an international standard commodity identification code and is designated so as not to be duplicated internationally, so the GTIN can be used as a key to ensure the identification of products.
Source of Funding
None declared.
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- Orii T. Contribution and development of medical safety by digitization of attached documents: How to verify the effectiveness and develop infrastructure. Med Care 2021;48:74–77.
- Suzuki R, Orii T, Yamaguchi T, Sugita M, Kato T. Utilization of barcodes to improve medical safety and operational efficiency – A review from incident reports. Kumamoto: 31st Annual Meeting of the Japanese Society for Medical and Pharmaceutical Sciences. 2021.
- Suzuki R, Yamaguchi T, Orii T, Sugita M, Kato T. Safe management of pharmaceuticals by utilizing standard barcodes (GS1). Nagoya: The 41st Annual Meeting of the Japanese Society for Medical Informatics. 2021.
- Pharmaceutical and Food Safety Bureau. Implementation Guidelines for Barcode Labeling of Ethical Drugs. Medical Affairs and Economic Affairs. 2016: 0830–0831.
- Hasegawa F, Ikeda S, Kaihara S. Survey Study on the Use of New Barcodes for Ethical Drugs in Medical Institutions. J Japanese Soc Healthc Hosp Adm 2009;46:177–185.
- Shiraishi H. Problems in the use of new barcodes for medical supplies in hospitals. Monthly Automat Recognit 2009;22:11–15.
- Yamakita K, Takasaki T, Umesato Y, Ohmichi H. A Study on the Effectiveness of Authentication System Using Injection Drug Barcodes in Preventing Medical Accidents. J Japanese Soc Healthc Hosp Adm 2011;48:13–22.